


The source document for this Digest states:
The releases of mercury to the biosphere can be grouped in four categories:
- Natural sources - releases due to natural mobilisation of naturally occurring mercury from the Earth's crust, such as volcanic activity and weathering of rocks;
- Current anthropogenic (associated with human activity) releases from the mobilisation of mercury impurities in raw materials such as fossil fuels – particularly coal, and to a lesser extent gas and oil – and other extracted, treated and recycled minerals;
- Current anthropogenic releases resulting from mercury used intentionally in products and processes, due to releases during manufacturing, leaks, disposal or incineration of spent products or other releases;
- Re-mobilisation of historic anthropogenic mercury releases previously deposited in soils, sediments, water bodies, landfills and waste/tailings piles.
The figure below shows these release categories with main types of possible control mechanisms.
The recipients of mercury releases to the environment include the atmosphere, water environments (aquatic) and soil environments (terrestrial). There are continuing interactions – fluxes of mercury – between these compartments. The speciation – the chemical form – of the released mercury varies depending on the source types and other factors. This also influences the impacts on human health and environment as different mercury species have different toxicity.
Given the understanding of the global mercury cycle, current releases add to the global pool of mercury in the biosphere – mercury that is continuously mobilised, deposited on land and water surfaces, and re-mobilised. Being an element, mercury is persistent – it cannot be broken down to less toxic substances in the environment. The only long-term sinks for removal of mercury from the biosphere are deep-sea sediments and, to a certain extent, controlled landfills, in cases where the mercury is physio-chemically immobilised and remains undisturbed by anthropogenic or natural activity (climatic and geological). This also implies that even as the anthropogenic releases of mercury are gradually eliminated, decreases in some mercury concentrations – and related environmental improvements – will occur only slowly, most likely over several decades or longer. However, improvements may occur more quickly in specific locations or regions that are largely impacted by local or regional sources.
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 77 to 80
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()
The source document for this Digest states:
The origins of atmospheric mercury deposition (flow of mercury from air to land and oceans) are local and regional as well as hemispherical or global. Several large studies have supported the conclusion that, in addition to local sources (such as chlor-alkali production, coal combustion and waste incineration facilities), the general background concentration of mercury in the global atmosphere contributes significantly to the mercury burden at most locations. Similarly, virtually any local source contributes to the background concentration – the global mercury pool in the biosphere - much of which represents anthropogenic releases accumulated over the decades. Also, the ocean currents are media for long-range mercury transport, and the oceans are important dynamic sinks of mercury in the global cycle.
The majority of atmospheric anthropogenic emissions are released as gaseous elemental mercury. This is capable of being transported over very long distances with the air masses. The remaining part of air emissions are in the form of gaseous divalent compounds (such as HgCl2) or bound to particles present in the emission gas. These species have a shorter atmospheric lifetime than elemental vapour and will deposit via wet or dry processes within roughly 100 to 1000 kilometers. However, significant conversion between mercury species may occur during atmospheric transport, which will affect the transport distance.
The atmospheric residence time of elemental mercury is in the range of months to roughly one year. This makes transport on a hemispherical scale possible and emissions in any continent can thus contribute to the deposition in other continents. For example, based on modelling of the intercontinental mercury transport performed by EMEP/MSC-E (Travnikov and Ryaboshapko, 2002), up to 50 percent of anthropogenic mercury deposited to North America is from external sources. Similarly, contributions of external sources to anthropogenic mercury depositions to Europe and Asia were estimated to be about 20 percent and 15 percent, respectively.
Furthermore, as mentioned, mercury is also capable of re-emissions from water and soil surfaces. This process greatly enhances the overall residence time of mercury in the environment. Recent findings by Lindberg et al. (2001) indicate re-emission rates of approximately 20 percent over a two-year period, based on stable mercury isotope measurements in north-western Ontario, Canada.
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 81 to 84
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()
The source document for this Digest states:
A large portion of the mercury present in the atmosphere today is the result of many years of releases due to anthropogenic activities. The natural component of the total atmospheric burden is difficult to estimate, although a recent study (Munthe et al., 2001) has suggested that anthropogenic activities have increased the overall levels of mercury in the atmosphere by roughly a factor of 3.
While there are some natural emissions of mercury from the earth’s crust, anthropogenic sources are the major contributors to releases of mercury to the atmosphere, water and soil.
Examples of important sources of anthropogenic releases of mercury:
Releases from mobilisation of mercury impurities:
- Coal-fired power and heat production (largest single source to atmospheric emissions)
- Energy production from other fossil carbon fuels
- Cement production (mercury in lime)
- Mining and other metallurgic activities involving the extraction and processing of virgin and recycled mineral materials, for example production of :
- iron and steel
- ferromanganese
- zinc
- gold
- other non-ferrous metals
Releases from intentional extraction and use of mercury:
- Mercury mining
- Small-scale gold and silver mining (amalgamation process)
- chlor-alkali production
- Use of fluorescent lamps, various instruments and dental amalgam fillings
- Manufacturing of products containing mercury, for example:
- thermometers
- manometers and other instruments
- electrical and electronic switches
Releases from waste treatment, cremation etc. (originating from both impurities and intentional uses of mercury):
- Waste incineration (municipal, medical and hazardous wastes)
- Landfills
- Cremation
- Cemeteries (release to soil)
On average around the globe, there are indications that anthropogenic emissions of mercury have resulted in deposition rates today that are 1.5 to 3 times higher than those during pre-industrial times. In and around industrial areas the deposition rates have increased by 2 to 10 times during the last 200 years.
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 85 & 94
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()
The source document for this Digest states:
There are significant uncertainties in the available release inventories, not only by source, but also by country. The best available estimates of mercury emissions to air from various significant sources are shown in the table below.
Table -Estimates of global atmospheric releases of mercury from a number of major anthropogenic sources in 1995 (metric tons/year). Releases to other media are not accounted for here.1
1 Note that releases to aquatic and terrestrial environments - as well as atmospheric releases from a number of other sources - are not included in the table, because no recent global estimates have been made. See chapter 6 for description of this issue.
2 Considered underestimated by authors of the inventory, see notes to table 6.10.
3 Represents total of the sources mentioned in this table, not all known sources. Sums are rounded and may therefore not sum up precisely.
4 Estimated emissions from artisanal gold mining refer to late 1980's/early 1990's situation. A newer reference (MMSD, 2002) indicates that mercury consumption for artisanal gold mining - and thereby most likely also mercury releases - may be even higher than presented here.
5 Production of non-ferrous metals releasing mercury, including mercury, zinc, gold, lead, copper, nickel.Continent Stationary combustion Non-ferrous metal production5 Pig iron and steel production Cement production Waste disposal 2 Artisanal gold mining4 Sum, quantified sources3 Europe 186 15 10 26 12 250 Africa 197 7.9 0.5 5.2 210 Asia 860 87 12 82 33 1070 North America 105 25 4.6 13 66 210 South America 27 25 1.4 5.5 60 Australia and Oceania 100 4.4 0.3 0.8 0.1 100 Sum, quantified sources, 19953,4 1470 170 30 130 110 300 1900 + 300 Based on references: Pirrone et al. (2001) Pirrone et al. (2001) Pirrone et al. (2001) Pirrone et al. (2001) Pirrone et al. (2001) Lacerda (1997) The emissions from stationary combustion of fossil fuels (especially coal) and incineration of waste materials accounts for approximately 70 percent of the total quantified atmospheric emissions from major anthropogenic sources. As combustion of fossil fuels is increasing in order to meet the growing energy demands of both developing and developed nations, mercury emissions can be expected to increase accordingly in the absence of the deployment of control technologies or the use of alternative energy sources. Control technologies have been developed for coal combustion plants and waste incinerators with the primary intention of addressing acidifying substances (especially SO2 and NOX), and particulate matter (PM). Such existing technologies may provide some level of mercury control, but when viewed at the global level, currently these controls result in only a small reduction of mercury from these sources. Many control technologies are significantly less effective at reducing emissions of elemental mercury compared to other forms. Optimised technologies for mercury control are being developed and demonstrated, but are not yet commercially deployed.
Available global estimates of atmospheric emissions from waste incineration, as well as other releases originating from intentional uses of mercury in processes and products, are deemed underestimated, and to some degree incomplete. However, recorded virgin mercury production has been decreasing from about 6000 to about 2000 metric tons per year during the last two decades, and consequently, related releases from mining and usage of mercury may also be declining.
Anthropogenic emissions from a number of major sources have decreased during the last decade in North America and Europe due to reduction efforts. Also, total anthropogenic emissions to air have been declining in some developed countries in the last decade. For example, Canadian emissions were reduced from about 33 metric tons to 6 metric tons between 1990 and 2000.
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 87 to 90
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()
| For details on: | See UNEP assessment: |
|---|---|
| The use of mercury for gold and silver extraction. | Chapter 6, section 1.3.4 |
The source document for this Digest states:
Regarding anthropogenic releases, the relative importance of intentional uses versus mobilisation of mercury impurities varies between countries and regions, particularly depending on:
- State of substitution of intentional uses (products and processes);
- Reliance on fossil fuels for energy production, particularly coal, and the presence of controls for other pollutants, which also reduce mercury emissions;
- Extent of mining and mineral extraction industry;
- Waste disposal pattern - incineration/landfilling;
- State of implementation of release control technologies in power production, waste incineration and various industrial processes.
For a number of countries, estimated contributions of intentional uses vary between 10 and 80 percent of the total domestic emissions to air, depending on the influence of the factors listed above. Rough estimates of distribution by main anthropogenic source types in each of these countries are shown in the chapter.
As an illustration, the figure below shows the overall turnover of mercury in the Danish society in 1992/93 in kilograms mercury/year (based on Maag et al., 1996). (Note that inputs and outputs in the figure do not balance because outputs reflect higher inputs from previous years. Net change in stocks was negative.)
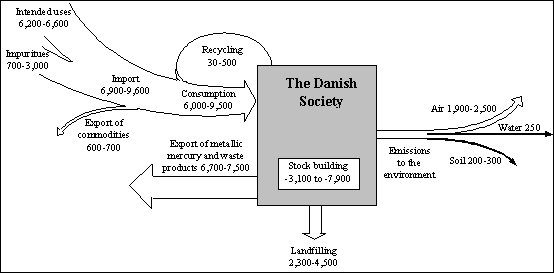
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 95 to 99
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()
The source document for this Digest states:
Natural sources include volcanoes, evaporation from soil and water surfaces, degradation of minerals and forest fires. The natural mercury emissions are beyond our control, and must be considered part of our local and global living environment. It is necessary to keep this source in mind, however, as it does contribute to the environmental mercury levels. In some areas of the world, the mercury concentrations in the Earth's crust are naturally elevated, and contribute to elevated local and regional mercury concentrations in those areas.
Today’s emissions of mercury from soil and water surfaces are composed of both natural sources and re-emission of previous deposition of mercury from both anthropogenic and natural sources. This makes it very difficult to determine the actual natural mercury emissions.
Published estimates of natural versus anthropogenic mercury emissions show significant variation, although more recent efforts have emphasized the importance of human contributions. Attempts to directly measure natural emissions are ongoing. Nonetheless, available information indicates that natural sources account for less than 50 percent of the total releases.
Source & ©: UNEP Global Mercury Assessment report, Summary of the Report,![]()
Chapter 6, paragraphs 91 to 93
For more information, see Chapter 6: Sources and cycling of mercury to the global environment![]()

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!