


Nanotechnology has rapidly promoted the development a new generation of smart and innovative products and processes, and have created a tremendous growth potential for a large number of industry sectors. Nanotechnology and engineered nanomaterials (ENMs) already have had a major impact on electronics, coatings, construction, food technology, the design of new materials such as nanocellulose applications, telecommunication, environmental technologies, medical technologies and drug development, nano-biocide applications and energy production as well as new agriculture, water purification systems and the utilization of solar energy among others. Nanomaterials and nanotechnology applications have also created some concerns about their possible effects on human health and safety and environmental burden.
Even though there is increasing amount of information of the hazard potential on several nanomaterials, there is a dramatic lack of systematic, and especially relevant, information on the potential hazards associated with these materials.
The current debate, including the lack of regulatory clarity, and the uncertainty surrounding the potential risks of nanomaterials have had a negative effect on the development, uptake and exploitation of nanomaterials in the European domain and have been identified as major barriers to innovation based on these technologies. To overcome these barriers, the development of a sound science-based foundation from which one can build a trustworthy and affordable safety framework is mandatory.
The goals of this document are to describe the current level of knowledge of the safety of nanomaterials and nanotechnologies, to identify knowledge gaps, and to set out concrete goals for the research on safety of ENM within the foreseeable future.
The European Commission is considering a new Action Plan for Nanotechnology, addressing the technological and societal challenges and strengthening the research and innovation efforts, with increased emphasis on sustainable development, competitiveness, and environmental, health and safety (EHS) issues.
In this context, the European NanoSafety Cluster is an initiative of the Research and Innovation Directorate General to bring together current research projects all across Europe that adress in a coherent and harmonized all aspects of nanosafety in its various dimensions, including a discussion forum and a dialogue with all stakeholders involved. About 100 scientists contributed to this report.
As public confidence in nanotechnology is crucially important if these products are to achieve commercial success, neutral and reliable communication and dialogue with the different stakeholders (regulators, industry, various interests groups, representatives of media and the public at large) associated with nanosafety can markedly enhance the acceptability of safe and trustworthy nanomaterials and associated technologies and promote a new safety culture in nanotechnologies. Dissemination of reliable information on nanosafety, and outreach to various stakeholder groups will all help assuring the general public and decision-makers that health and environment aspects are being taken into account
The goals of this document are to describe the current level of knowledge of the safety of nanomaterials and nanotechnologies, to identify knowledge gaps, and to set out concrete goals for the research on safety of ENMs within the foreseeable future.
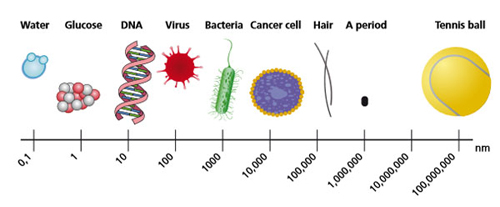
The usual definition of nanometerial is simply something that has a size in the range of nanometers (usually 1-100nm), but this does not reflect the diverse nature of nanomaterials. The definition used here is the one that the European Commission adopted in 2011 :a ‘nanomaterial’ is: a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm-100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50 % may be replaced by a threshold between 1 and 50 %. »
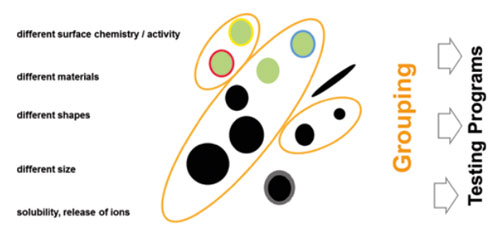
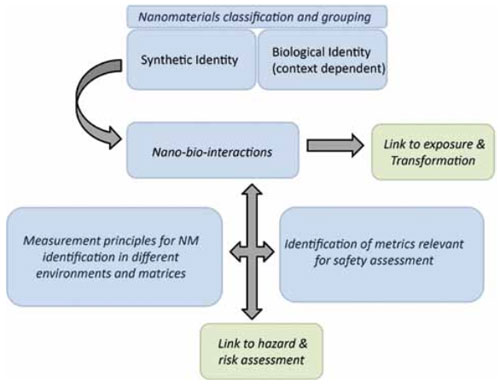
A gap in terms of classifying nanomaterials is that there are multiple different variants of each type of nanomaterial, all of which may differ in terms of their impacts. Thus, there is a critical need for an – International Union of Pure and Applied Chemistry (IUPAC)-type approach to naming and describing nanomaterials.
In order to enable prediction of impacts, a classification based on key physicochemical parameters (metrics) or biological interactions were identified in the report and should be adopted. It is for exemple suggested that a distinction should be made between the synthetic and biological identity of nanomaterials.
The crucial challenge in all cases is to identify the harmful agents and to differentiate them from their innocent counterparts so that the appropriate regulatory decisions can be made to protect human health and the environment. The understanding of how nanomaterials interact with living system is incomplete and, thus, we are not yet in a position to know what to look for exactly when testing the toxicity of nanomaterials.
The majority of ENMs may be harmless or only modestly harmful, but there is a plethora of evidence revealing that many of the materials may be highly harmful. Nanomaterials can be coated by all kinds of substances, either deliberately (coating) or unintentionally (surface layer), and very little is known about the impact of the resulting surface chemistry on both the toxicokinetics (absorption, biodistribution and clearance) and toxicodynamics (toxicity and recovery). Currently, there is no direct evidence of the presence of groups of population highly vulnerable to nanomaterial exposure. Their presence is suggested by epidemiological data concerning the health impacts of the ultrafine component of air pollution, but some experimental data regarding nanomaterials are now becoming available. Exposure to ultrafine particles of air pollution exacerbates pre-existing asthma and chronic obstructive pulmonary disease, increases inflammation and airway acidification. The real concern is the lack of systematic studies on hazards of or exposure to nanomaterials.
Apart from oral and dermal exposure, inhalation is considered the most relevant exposure route for potential risk. Special considerations are given regarding susceptible populations and vulnerable conditions including cardiovascular diseases, allergic diseases and asthma, pregnancy, elderly and babies. The report assumes that by 2020, the main toxic mechanisms of various types of ENMs will be understood and sound criteria to classify nanomaterials for toxicity will exist.
Regarding the potential environmental impact of nanomaterials, implementation of standardized ecotoxicological tests for their effects in the aquatic environment may be problematic as the tendency of nanoparticles to form clumps changes with the concentration in water. Since there is evidence for delayed effects of exposure to nanomaterials, future work should consider long-term effects in the aquatic environment. The research on the terrestrial environment needs to focus on long-term effects in realistic concentrations and ecologically relevant environmental conditions.
All nanoparticles in chemical substances must meet the requirements of the European Union REACH (Registration, Evaluation and Authorization of Chemicals) Regulation (1907/2006). Also, the biocidal product regulation (Regulation (EC) 528/2012), which is in force since September 2013, provides a framework of rules that apply to the marketing of biocidal products including nanomaterials substances and products (Directive 98/8/EC). The current view is that the general existing regulatory frameworks are applicable but have to be adapted and extended for some ENMs specific issues. It has been emphasized that ENMs are the subject of some special properties, especially those related to the transformation of materials during their life-cycle (LCA) or after their release into the environmental compartments which are known to alter their relevant substance characteristics e.g. size, shape, charge, state of agglomeration etc.
However, the available laboratory tools for the assessment of the safety of engineered nanomaterials are often inappropriate, or so laborious that adequate safety assessment remains highly problematic. We still lack a fundamental understanding of how nanomaterials interact with living systems and, thus, we are not yet in a position to assess the relevant end-points for nanomaterial toxicity.
Assessing true workplace exposure may be challenging to measure in some cases because of lack of understanding of the predictive value of different metrics in predicting human hazard and risk. Today, the most urgent challenge related to nanomaterials and nanotechnologies is how can we gather the essential knowledge that could be utilized for reliable risk assessment and adequate risk management and governance.
Current resources or test methods are not likely to be sufficient to enable safety assessment of the numerous novel nanomaterials that are emerging at an ever increasing pace. This means that new safety assessment paradigms need to be developed during coming years to solve this problem. We still lack a fundamental understanding of how nanomaterials interact with living systems and, thus, we are not yet in a position to assess the relevant end-points for nanomaterial toxicity. We are faced indeed with a large number of new materials for which testing or screening of toxicity is required. We are still dealing with first generation of nano-enabled products but it is likely that we will soon be confronted by the second generation products containing active nanostructures, and then to third generation systems of integrated nano-systems and, finally, by the year 2020 according to some predictions, to fourth generation products or heterogenous molecular nano-systems that allow the manufacture of molecular devices ‘by design’. This means that methods for assessment of the safety of next generation nano-enabled products also must evolve: nanotechnology is a moving target and researchers in the nanosafety field cannot afford to be aiming at a target that no longer exists.
There is therefore an urgent need for a “new” toxicology for the 21st century to resolve this situation and innovative methods for prediction of nanomaterial toxicity are needed. New toxicology should produce a shift from toxicity testing primarily in animal models to in vitro assays and in vivo assays using lower model organisms, along with computational modeling, thus enabling the evolution of toxicology from being an observational science into a predictive science. By 2020, the ultimate goal will be to develop a computational tool that can predict ENMs safety based on the evaluation of minimal but sufficient amounts of information generated by in vitro and in vivo studies, to provide a robust safety classification. It is a tool that is beyond the current technological capabilities, but when it becomes available it will promote the utilization of safety-by-design principle, and also be capable of improving the speed of hazard identification and risk assessment.
Further, epidemiological studies are a key tool for assessing whether exposed populations are safe at given levels, to evaluate the relevance of toxicological findings to human health and to identify potential biological effects that had not been predicted on the basis of toxicity studies. As workers are likely to have earlier and higher exposures to ENMs than the general population, they represent a good target for conducting the first epidemiological studies.
This situation called the rapid identification by the Nanosafety Cluster of four main research needs and priorities for the coming 10 years:

One of the main obstacles in studying consumer exposure is how to achieve a reliable measurement of particles in the different matrices of consumer goods and food products. Strategies to overcome this limitation will need to be developed. The major obstacle in studying the release, transformation and exposure of nanomaterials is the identification of the particles themselves. Discrimination of particles by type (e.g. engineered vs. natural vs. particles produced during the manufacturing process itself) is of importance when assessing exposures, and in subsequent analyses that interface with health studies.
One possibility to put them in practices would be to develop a single-site highly equipped facility with capacities to serve other EU nanosafety research facilities in strategic research areas in a stable organization with guaranteed fundamental resources into the foreseeable future.
Another possibility, which would be potentially realistic at the European Union level, would involve networking of high-quality nanosafety research organizations, i.e. organizations with suitable space and laboratories, research equipment, human resources and competences, national stable funding and existing administrative support in research organizations within European Union Member States.
Other crucial issues include a strong commitment from regulators to promote standardization and the involvement of industrial partners that are willing to adopt the safety by design approach in their business thinking. An important building block to facilitate the safe implementation of nanotechnologies is research in support of regulation, and its implementation is crucial for strongly promoting nanosafety within EU in the near future. This research is intended to provide science based regulatory approaches to ensure societal acceptance of nanotechnologies and good understanding between authorities, industry and society about risks and their management.
To avoid unnecessary duplications, the US-EU dialogue, “bridging nano-EHS research”, engages in an active discussion on nano-EHS issues to encourage joint programs of research, establish communities of practice between researchers and corresponding funding sources to enable collaborations. The Organisation for Economic Cooperation & Development (OECD) and in particular the OECD Working Party on Nanotechnology (WPN) is in a central role in the promoting of development joint global initiatives on responsible development and applicability of nanotechnology between OECD Member States, OECD Observer States and other stakeholders.
As nanotechnology is an “enabling technology”, it is a key element in the innovation/value chain that has a tremendous potential to provide answers to societal solutions, it is therefore of critical importance to incorporate nanosafety into the development of novel nanotechnologies and products safety before design.
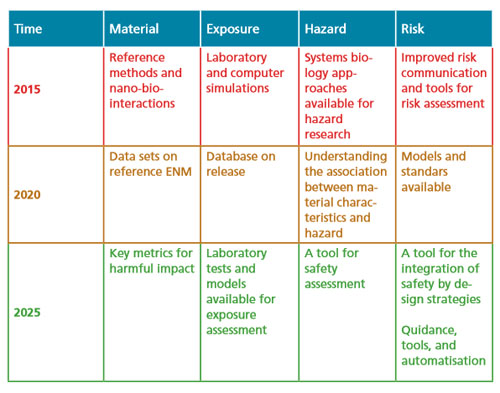
Therefore the sustainable implementation of nanotechnologies relies on the successful and timely implementation of the roadmap proposed in this report - which is subject to further refinements as new research priorities emerge – to lead to the development of a tool box for exposure assessment, for hazard prediction, and for risk assessment and prediction as well as their management.
The research roadmap proposed here aims at providing directions towards a sustainable development of nanotechnology- based tools and products. It is based on the premises that a level of generalised knowledge in the different areas mentioned and dealt with above shall be achieved within the next 10-15 years and this will mean that new materials will be safer by design and this philosophy will be beneficial.

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!