


PCBs, or polychlorinated biphenyls, are a class of man-made chemicals.

The commercial production of PCBs started in 1929 but their use has been banned or severely restricted in many countries since the 1970s and 80s because of the possible risks to human health and the environment.
As PCBs are resistant to acids and bases as well as to heat, they have been used as an insulating material in electric equipment, such as transformers and capacitors, and also in heat transfer fluids and in lubricants. PCBs have also been used in wide range of products such as plasticizers, surface coatings, inks, adhesives, flame-retardants, paints, and carbonless duplicating paper.
Since 1929 around 2 million tonnes of PCBs have been produced, about 10% of which still remain in the environment today.
Sources of PCB pollution:
This text is a summary of: IPCS - WHO
Polychlorinated biphenyls : Human health aspects. Concise international chemical assessment document 55
Section 1 Executive summary and Section 4, Sources of human and environmental exposure![]()
PCBs, or polychlorinated biphenyls, are a class of man-made organic chemicals. Each PCB molecule contains two phenyl rings. A phenyl ring is a ring of 6 carbon atoms to which hydrogen atoms are attached. In PCBs, chlorine atoms replace some of these hydrogen atoms.
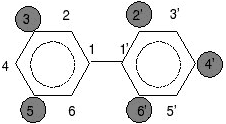
Chlorine atoms may be present at some or all of the 10 possible positions which are numbered 2–6 on one ring, and 2'–6' on the other ring (see figures below). In total, 209 different PCBs can be formed. These different combinations are called congeners, each having a specific number of chlorine atoms located at specific positions.
One example below shows a PCB with five chlorine atoms, two chlorine atoms at the 3 and 5 positions on one ring and three chlorine atoms at the 2’, 4’ and 6’ positions on the other ring. The other example shows a PCB with chlorine atoms at the 2 position on one ring, and the 2’ position on the other.
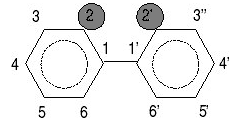
Two different systems are used for naming PCBs. In the IUPAC system used in the examples above, the numbers at the beginning of the name specify the sites where chlorines are attached to the phenyl rings. Another system assigns a separate number, from 1 to 209, to each of the 209 specific PCB congeners. (see figures to the right)
(see conversion table)
PCBs which have the same number of chlorine atoms attached but at different positions, are referred to as isomers.
The two rings in a PCB molecule can rotate around the bond connecting them. The shape of the molecule is further influenced by the repulsion between nearby chlorine atoms so that the rings of a specific PCB will either lie approximately in the same plane (called coplanar) or in different, more perpendicular planes (termed non-planar). Those PCBs which have both rings lying in the same plane are considered to be most toxic, based on combined health effects considerations. They are also referred to as "dioxin-like". (see question 3)
PCBs were manufactured and sold as mixtures of several congeners, with a variety of trade names, including Aroclor, Pyranol, Pyroclor (USA), Phenochlor, Pyralene (France), Clophen, Elaol (Germany), Kanechlor, Santotherm (Japan), Fenchlor, Apirolio (Italy), and Sovol (USSR).
Physical properties of PCBs:
Although in general PCBs do not easily evaporate, especially those with more chlorine atoms, PCB evaporation does occur, and can account for significant amounts of PCB transport (see question 2.1)
PCBs can be measured in:
This text is a summary of: IPCS - WHO
Polychlorinated biphenyls : Human health aspects. Concise international chemical assessment document 55
Section 2, Identity and physical/chemical properties ![]()
Section 3, Analytical methods![]()

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!