

Sprachen:

The main origin of antibiotic resistance, also called antimicrobial resistance , is their misuse. As underlined by the European Centre for Disease Prevention and Control (ECDC) they are three main types of misuses (ref 1):
Antimicrobials are defined as medicinal products that kill or stop the growth of living microorganisms. Besides the “antibacterials”, usually called antibiotics, because these are active against bacterial infections, these include also among others:
• Antimycobacterial drugs (which are antibacterials specifically active against tuberculosis and other mycobacterial infections);
• Antivirals (active against viral infections, e.g. influenza, HIV, herpes infections);
• Antifungals (active against fungal infections);
• Antiparasital drugs (active against malaria and other infections due to parasites).
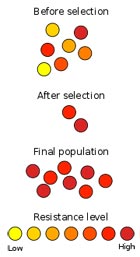
Although some bacteria are naturally resistant to certain antibiotics (intrinsic or inherent resistance), others acquire an antibiotic resistance, which is caused by mutations in some of their genes and make that specific antibiotics lose their ability to kill or stop bacterial growth. Furthermore, the antibiotic resistance acquired by one species of bacteria can easily spread to other bacterial species because bacteria easily exchange their genetic material.
Because antibiotic resistance is increasing, the problem is now a major public health threat.
In particular, in European hospitals and communities, the resistance to major antibiotics of common bacteria such as Escherichia coliwhich causes urinary tract and more serious infections but also for Staphylococcus aureus (MRSA or methicillin-resistant Staphylococcus aureus ), Klebsiella pneumoniae and Pseudomonas aeruginosa. A recent WHO report (ref 3) makes a clear case that resistance to common bacteria has reached alarming levels in many parts of the world indicating that many of the available treatment options for common infections in some settings are becoming ineffective. The report summarizes the situation in major disease-specific control programmes (i.e. HIV, influenza, malaria and TB) and in related fields (i.e. foodborne and fungal infections).
By consequence, there are now situations where infected patients cannot be treated adequately because the responsible bacterium is totally resistant to available antibiotics. This resistance may delay getting the right or even any treatment to patients and may result in complications, including death. A patient may need more care as well as alternative and more expensive antibiotics, which may have more severe side effects.
Also, treatment of antibiotic-resistant bacteria may also require intravenous antibiotics given in hospitals instead of oral antibiotics that could be taken by patients at home. This means that progress in modern medicine, which relies on the availability of effective antibacterial drugs, is now at risk, says the WHO report, giving some examples :
In the US, the Centres for Diseases Control (CDC) estimates that in the United States, more than two million people are sickened every year with antibiotic-resistant infections, with at least 23,000 dying as a result. The estimates are based on conservative assumptions and are likely minimum estimates. They are the best approximations that can be derived from currently available data.
Further, few new antibiotics have been discovered since 1985 and few marketed in recent years : the pipeline for the development of new antibacterial drugs is now virtually empty, states the recent WHO report.
Bacteria causing a wide range of infections may become resistant to multiple antibiotics : urinary tract infection, pneumonia, skin infection, diarrhoea, bloodstream infection. The high proportions of resistance to 3rd generation cephalosporins reported for E. coli and K. pneumonia, for example means that treatment of severe infections likely to be caused by these bacteria in many settings must now rely mainly on carbapenems, the last resort to treat severe infections in communities and hospitals by this bacteria and as long as these do not also become resistant to carbapenems. These antibacterials are more expensive, may not be available in resource-constrained settings, and of great concern is the fact that K. pneumoniae resistant also to carbapenems has been already identified in most of the countries that provided data.
Regarding turberculosis, globally, 3.6% of new cases and 20.2% of previously treated cases are estimated to have be multidrug resistant with much higher rates in Eastern Europe and central Asia. In the case of malaria, spread of artemisinin-resistant strains, or the independent emergence of artemisinin resistance in other regions, could jeopardize important recent gains in malaria control.
Patients in hospitals are at special risk for infections by bacteria present in the hospital and thus unrelated to the reason for admission. These so-called “nosocomial infections” or, more generally, “Healthcare-associated infections” can involve some pathogenic bacteria that developed resistance to antibiotics, like the Staphylococcus aureus resistant to methicillin, an antibiotic representative of those which are usually effective against it, Enterobacteriaceae producing enzymes able to destroy some antibiotics and responsible of bloodstream infections, Enteroccocci resistant to vancomycin causing heart valve infections or Acinetobacter baumannii resistant to carbapenems producing surgical site and wound infections.
The first challenge to solve is the significant gaps in surveillance of antibiotic resistance, and the lack of standards for methodology, data sharing and coordination. Antibiotic resistance is still largely under-reported, compromising control efforts, says the WHO report, due for one part to this lack of global consensus on methodology and data collection.
However, it is observed that countries with lower resistance rates have generally lower use of antibiotics, whereas countries with higher antibiotic resistance rates use more antibiotics. In Europe, there is a gradient North-South with low rates in Scandinavian countries and the Netherlands and high rates in Southern Europe.
Anyway, in 2001, the WHO already issued a global strategy and guidelines to help countries in setting up systems to monitor antibiotic resistance and to implement efficient actions (ref 3). The same year, the Council of the European Union issued a recommendation asking countries to put in place actions to ensure prudent use of antibiotics and some countries which launched national programs, including public awareness campaigns, several years ago have observed a decrease in both consumption of antibiotics and in antibiotic resistance. This led to the creation of a European Antibiotic Awareness Day, a campaign to reduce use of antibiotics in situations where they are not necessary, for example for viral infections such as colds and influenza.
Nowadays, many of the most immediate and urgent concerns still relate to antibiotic resistance in common bacteria. In line with the WHO recommended strategy, the European Centres of Disease Control (ECDC) considers that three strategic areas of intervention that should be prioritized (ref 1):
Among WHO regions (ref 2), the greatest country-level data were obtained from the European Region. For the Region of the Americas, the Latin American Antimicrobial Resistance Surveillance Network (ReLAVRA) was created in 1996 and since then increased its ability to detect, monitor and manage data on antibiotic resistance and generated data for decision making, based on the growing number of countries participating in the network. In the US, CDC initiatives include four core actions that fight the spread of antibiotic resistance (ref 4). In the African Region, surveillance of drug resistance is carried out in only a few countries and systematic efforts to collect data on antimicrobial resistance have not yet been undertaken in the South-East Asia Region. The collaboration established In the 1980s among 14 States in the Western Pacific Region to share findings for more than 20 key hospital was unfortunately interrupted in 2000 for other priorities.
The major cause of antibiotic resistance in humans remains the use of antibiotics in human medicine. However, since antibiotics used to treat and prevent bacterial infections in animals belong to the same chemical groups as those used for humans, animals may acquire bacteria that are resistant to antibiotics also used against human infections.

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!