

Sprachen:

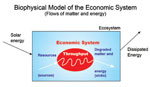
According to the principles or "laws" of thermodynamics6, in an isolated system that exchanges neither energy nor matter with the outside, energy is conserved (first law). Within such a system, any transformation of matter or energy is accompanied by the irreversible dissipation, called entropy, in a dispersed and disordered form of a part of this energy under the form of heat (2nd law). In this context, the Earth is not an isolated but a closed system where energy enters and leaves but material does not (except for the rare meteorites), as it is the case in an open system.
In such a closed system, structures can spontaneously appear
and organize, the so-called "dissipative" structures that will
favor and even maximise the dissipation of the energy that
passes through the system or
accumulates there7.
This local state of spontaneous self-organised being charactru-ised by a lower state of entropy. The
maintenance of their existence is therefore linked to that of
the flow of energy which "nourishes" them. The simplest example
is that of the hurricane, a structure which, beyond a critical
threshold, is
organized locally and dissipates the heat accumulated by the
ocean towards the
atmosphere.
Biological systems are only more sophisticated forms of
dissipative structures .
(See as a short introductive animation video on the subject: The principle of emergence
www.youtube.com/watch?v=_WLYOYE8a5o![]()
But the constraints of the second law of thermodynamics, that of entropy, mean that the maintenance of such organized structures requires a continuous supply of energy and matter. This imposes a closed system like the Earth that does not exchange matter with the outside, the recycling of it (water in the case of the hurricane, water CO2 and minerals in the case of plants, plants in the case of animals, …). In the loops mentioned above and characterizing the (eco) systems' operation, the recycling of materials is sometimes compared to the gathering of the beads of a broken collar scattered on the ground: in limited quantities and not renewable, their loss can lead to the depletion of the resources necessary to maintain the organized dissipative system.
To avoid such a global negative entropy balance in our organised dissipative systems, including biological ecosystems and human societies, there is therefore an absolute need to optimize it by reducing, reusing and recycling (the 3 "R") in a circular economy the products and services we use and thus conserving the natural non-renewable resources. This implies integrating quantitatively into the calculation of the overall value of a product or service all its environmental or environmental 'externalities': material and energy, societal and economic.
6 See for example: https://en.wikipedia.org/wiki/Laws_of_thermodynamics![]()
7 Axel Kleidon - Thermodynamic Foundations of the Earth System ![]()

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!