

Langues:

There is increasing concern in the general public about the potential toxic effects of chemical mixtures (in the media often referred to as “cocktail-effects”). Current legislation at EU level requires, only in a few instances, the assessment of cumulative risks from the exposure to multiple chemicals (e.g. for pesticides when suitable methodology is available).
Humans and ecosystems are indeed continually exposed to a very complex mixture of chemicals the composition of which is always changing. However, in the great majority of current risk assessments only a single chemical is considered and there are no generally applicable guidelines as to when and how assessment of combinations of chemicals should be carried out. It has became necessary to evaluate whether new EU guidelines should be developed for the assessment of chemical mixtures and the regulatory framework be strengthened.
This opinion does not address pharmaceuticals and essential metals and nutrients for human health. Furthermore, it does not address metals for environmental combination assessment because these assessments require the use of specific approaches, e.g. essentiality, background concentrations, bioavailability.
Mixtures of chemicals are considered to be:
Two cases should be considered :
Substances acting via a similar mode of action
For exposure to chemicals with common specific modes of action, there is strong evidence that produce additive effects which are larger than the effects of each mixture component applied singly. In the case of such combinations of chemicals that interact with the same sub-system of an organism, the concept of dose addition is thus applicable for the prediction of their effects when the toxicities of individual components are known. Dose additivity is assumed over the entire dose range, including doses/concentrations below the individual No Observed Adverse Effect Levels/ or Concentrations (NOAEL or NOAEC/Cs) of the mixture components.
Deviations from predicted additivity, indicative of synergic or antagonistic effects, are comparatively rare, relatively small and largely confined to mixtures with only a few compounds. A review of scientific literature for deviations from expected additivity found that - in toxicology studies in humans and other mammals - such deviations “were observed quite rarely”.
This currently available scientific evidence as well as pragmatic considerations, support the idea of adopting dose addition as the preliminary default concept for the assessment and prediction of mixture effects. This approach, said a report of the University of London to the EU DG Environment in 2009 , is borne out by current practice in many regulatory bodies in the EU, USA and by recommendations of international bodies. The use of a dose addition method is recommended in the ECHA guidance document within the REACH Regulation in the EU.
Substances acting via different modes of action
With mixtures composed of chemicals with diverse modes of action, there is, according to the same report, decisive evidence that the effects are higher than those of the individual components present at doses equal to, or below a no-effect level. This is derived from studies relevant to human toxicology and to ecotoxicology that have been conducted with such mixtures. Since information on human populations and wildlife is currently missing, according to the report, the uncertainty or “margin of safety “ factors used in risk assessment made for single chemicals could presently not offer sufficient room to take into consideration the effects for all possible realistic mixtures.
The Opinion of the Scientific Committees is that, for chemicals acting independently, no robust evidence is available and it is very unlikely (see pg 13 of the Opinion) that exposure to a mixture of such substances is of health concern if the individual chemicals are present at or below their zero-effect levels.
Indeed, interactions (including antagonism, potentiation, synergies) usually occur at medium or high dose levels as compared to the lowest effect levels. At low exposure levels, they are either not occurring or toxicologically insignificant. ) If no mode of action information is available, the dose/concentration addition method should be preferred over the independent action approach. Prediction of possible interaction requires expert judgement and hence needs to be considered on a case-by case basis.
The scientific state of the art of mixture toxicology shows that mixture risk assessment is indeed necessary, in order to avoid underestimations of risks that might occur under the current approach considering the risk of effects on a chemical-by-chemical basis. In the meantime, it appears also that such risk assessments of mixtures is possible with reasonable accuracy and precision, as demonstrated by a multitude of risk assessment methods already in use by international bodies & EU member states. In particular, there is no need for the experimental testing of each and every conceivable mixture, which would indeed make risk assessment unmanageable.
Except for mixtures composed of substances with a similar mode of action, current evidence does not show significant mixture toxicity at exposures at or below zero-effect levels of the individual components. It is important however to underline that “no-(adverse)-effect levels” or “concentrations” (NO(A)ELs and NO(A)ECs) identified from experimental studies do not always represent zero (no)-effect levels.
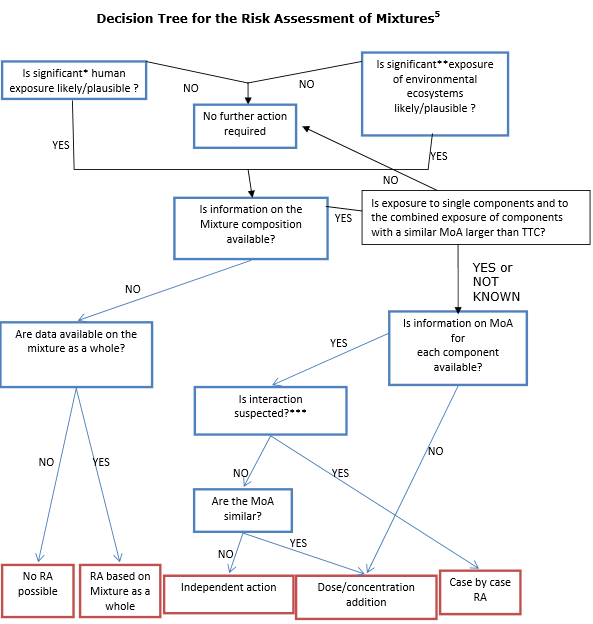
Based on its conclusions, a decision tree for evaluating the risk of chemical mixtures is proposed by the Committees.
1 SCHER, SCCS, SCENIHR, ![]()
Some researchers focussed on the combination effects of substances interfering with the endocrine system, in particular oestrogen-receptor binding compounds and their potential to synergise in combination. However, the relative potencies of o,p’-DDE (a metabolite of DDT), PCBs, nonylphenol, and dieldrin as compared to those of oestradiol are about a million-fold lower than that of estradiol. In 2007, concentrations in the blood of German, US and Japanese pregnant are about 570 to 5,800 fold below that of oestradiol and the highest value of 18.9 μg/L is still about 125 times lower than that of oestradiol. From this it was concluded that an interaction of the compounds at the receptor with physiological consequences is unlikely.
The general principles of mixture toxicology are applicable in ecotoxicology for predicting effects at population level. However, the concepts of “independent action”, “dose/concentration additions” and “synergistic action” should also be investigated at the population level (in addition to the individual and sub-individual).
At the community level, an additional concept of “synergism” is also possible, considering the combined effects of different chemicals on different taxonomic groups and the indirect consequences on the structure and functioning of the community. The assessment of the complex consequences at the community level cannot be performed using only toxicology-based approaches. It requires approaches based on ecology that accounting for interactions and indirect effects.
For ecological effects, the exposure to mixtures of dissimilarly acting substances at low, but potentially relevant concentrations should be considered as a possible concern, even if all substances are below the individual PNECs (Predicted no effect concentrations). Consequently there is a need for improving the current knowledge and methodologies, and developing holistic approaches for the ecological risk assessment of chemicals under realistic conditions.
When it comes to the question of whether the toxic effects of a mixture can be predicted, it has to be realised that this is only possible under the condition that the individual mixture components are fully identified and that their mode of action, as well as dose response curves, are known or appropriate assumptions can be made. This is a situation which, in human toxicology as well as in ecotoxicology, is rarely given.
A major knowledge gap at the present time is the lack of knowledge on where, how often and to what extent humans and the environment are exposed to certain chemical mixtures and how exposure may change over time. There is a need to better understand human and environmental exposures, both through the use of monitoring and modeling (Tornero-Velez et al. 2011).
For many chemicals, there is no good information on their mode of action. Currently there is neither an agreed inventory of modes of action, nor a defined set of criteria on how to characterise or predict a mode of action for data-poor chemicals or how to group chemicals into assessment groups. Interactions of chemicals in mixtures are also difficult to foresee, particularly for long-term effects. Research is needed to define criteria that predict potentiation or synergy.
In ecotoxicology, the problem is even more complex. A knowledge of all possible modes of action that may occur in the different types of organisms of a complex biological community is difficult (if not impossible) to be attained. On the other hand, it must be considered that ecologically relevant endpoints are generally broader and not so specific (e.g. toxicity on specific organs, etc.) as in human toxicology.
REFERENCE:
SCHER, SCCS, SCENIHR, ![]()
OTHER REFERENCES:
WHO OECD ILSI/HESI -
International Workshop on Risk Assessment of Combined Exposures to
Multiple Chemicals Workshop Report Series on Testing &
Assessment No. 140 February 2011, Paris, France ![]()
Swedish Chemicals Agency - ![]()
ECETOC - ![]()

This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!